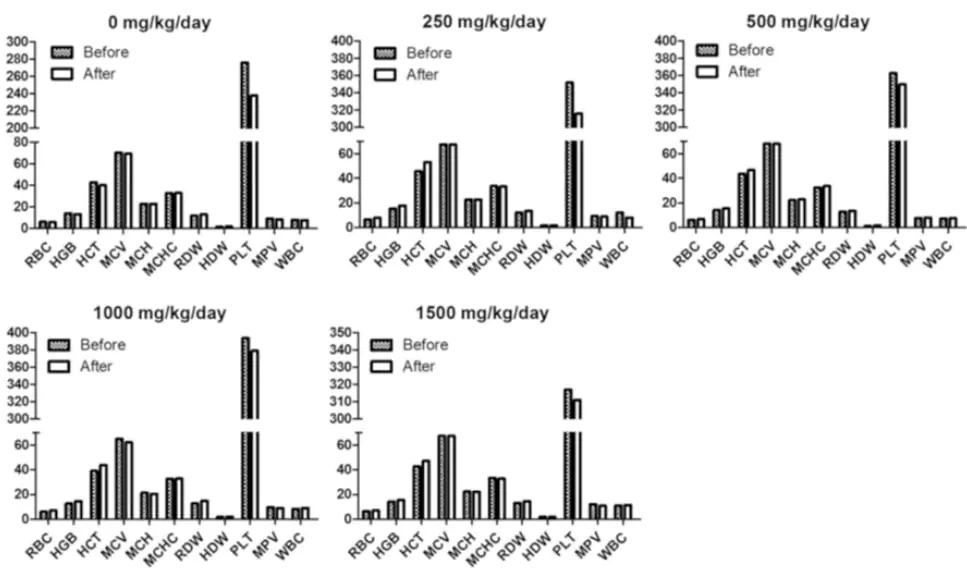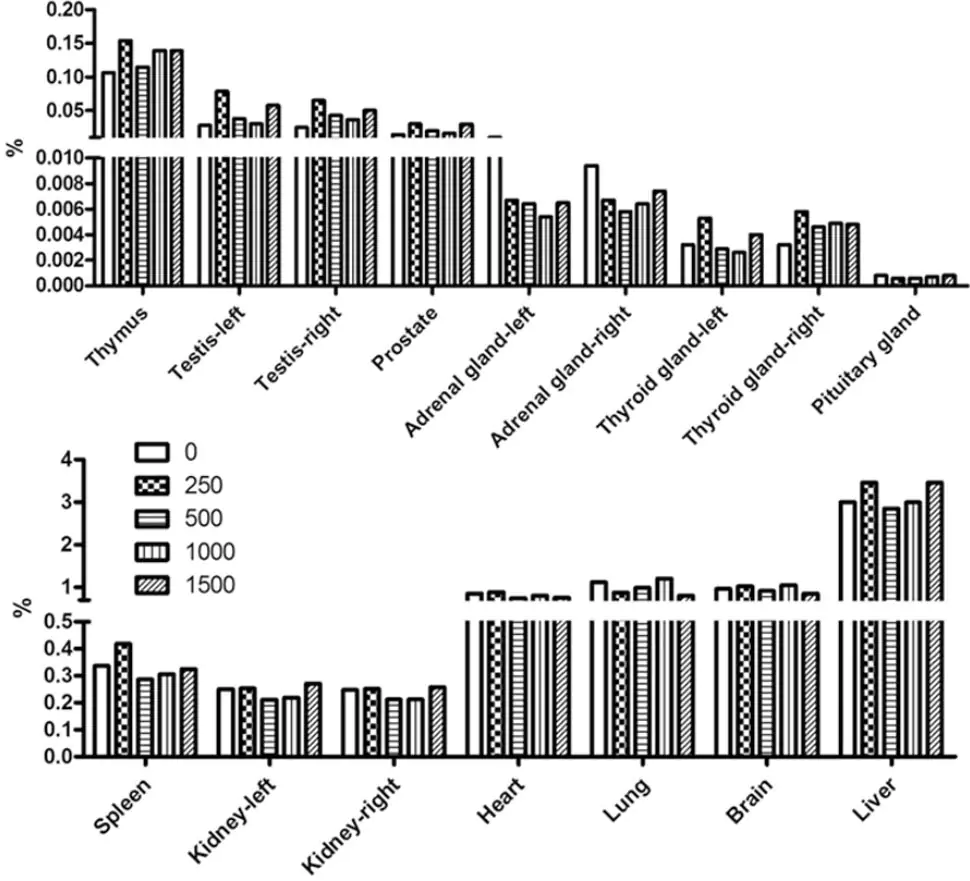

| Product Name | Drug Compound | Usage/Dosage | Active Mechanism | Side-Effects |
| Razadyne tablet (4, 8mg) Razadyne PR SR capsule (8, 16, 24mg) | Galantamine | 2 times/day for 4 weeks, 4 mg/dosage; 2 times/day after 4 weeks, 8 mg/dosage (maximum dosage of 24mg/day) | Acetylcholinesterase (AChE) inhibitor | Nausea, vomiting, diarrhea, stomachache, indigestion, loss of appetite, fatigue, dizziness, headache, drowsiness, weight loss |
| Exelon capsule (1.5, 3, 4.5, 6mg) | Rivastigmine | 2 times/day, 1.5mg/dosage; Titration up to 3, 6, 9, 12mg/day in 2-week intervals (maximum dosage of 12mg/day) | Acetylcholinesterase (AChE) inhibitor | Nausea, vomiting, diarrhea, stomachache, loss of appetite, dizziness, agitation |
| Aricept tablet (5, 10, 23mg) | Donepezil | 5mg/dosage once a day (for over 4 weeks); maintain a 5-10mg dosage per day | Acetylcholinesterase (AChE) inhibitor | Nausea, vomiting, muscle cramps, fatigue, vertigo, diarrhea |
| Namenda tablet (10mg) Namenda oral drops (10mg/g) | Memantine | 10mg/day; gradually increase dosage by 10mg/day in 1 week intervals; spread dosages of 20-30mg evenly over the course of the day (maximum dosage 60mg/day) | NDMA receptor antagonist | Dizziness, confusion, sleep disorder, agitation, fatigue, headache, constipation |

| Project Milestones & Timeline | |
| Project Code: | PM012 - (650mg/tablet of seven extracts that include rehmannia root) |
| Type of Drug: | Natural product, Tablet (coated), Oral administration |
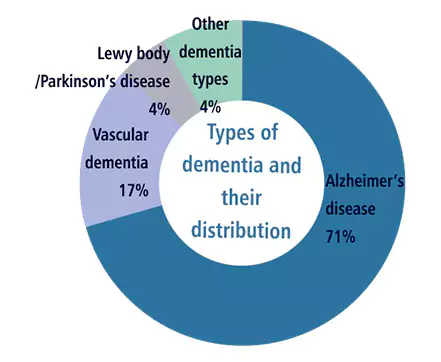
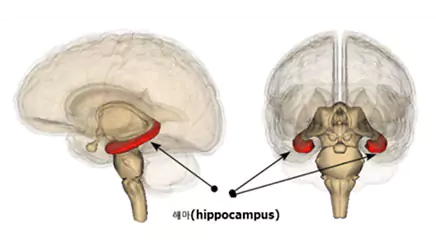
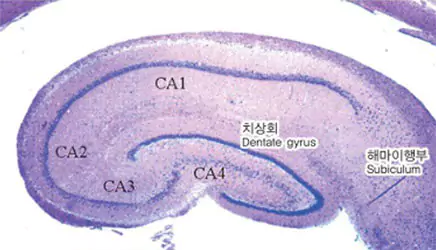
| Indication: | Dementia (Alzheimer's disease) |
| Target: | Clinical trials (local and global) |
| Control Group | Active Comparator Group | Test Group |
| Saline solution | Donepezil 0.6mg/kg | PM-012 100mg/kg, PM-012 400mg/kg |
| 12 weeks | 12 weeks | 12 weeks |
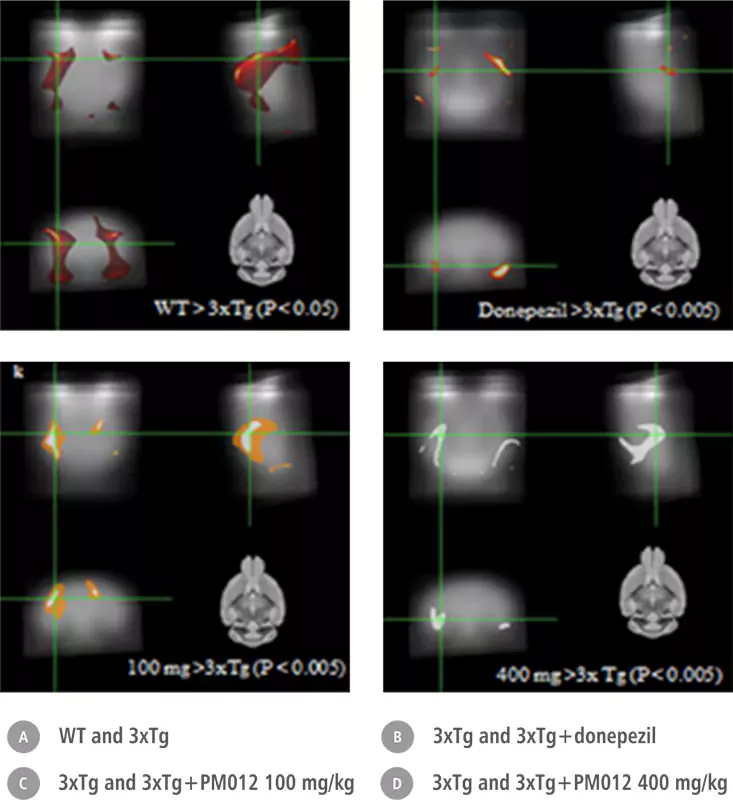
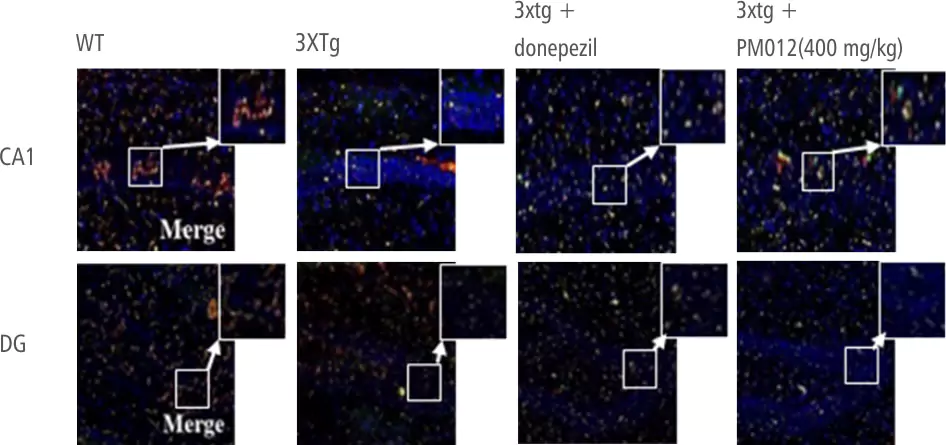
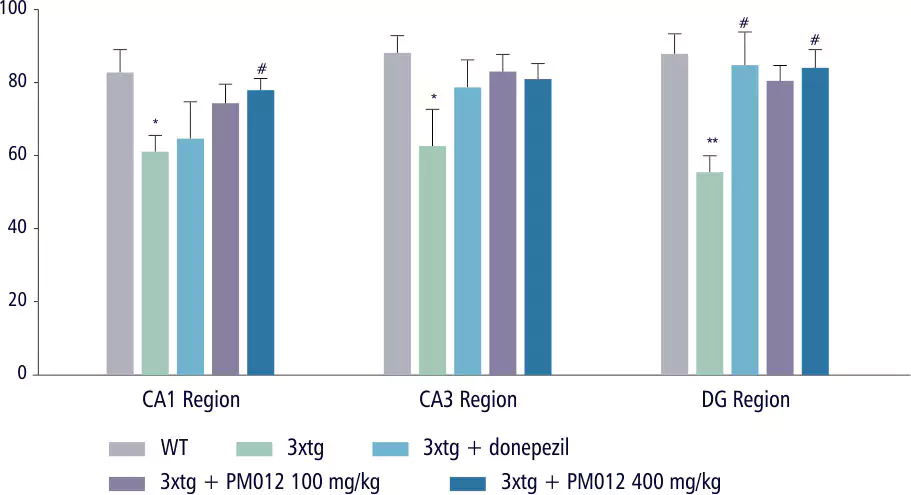
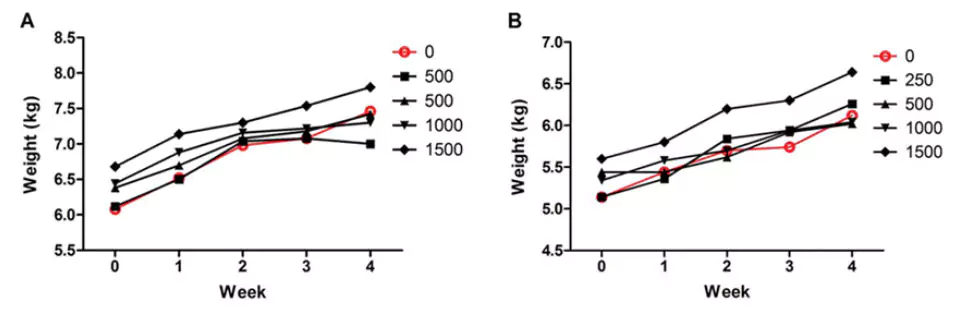
| Control Group | Ginko Biloba | Soya Lecithin | PM-012 |
| Only drinking water | 2.6mg/100g (weight) daily | 100mg/100g (weight) daily | 400mg/100g (weight) daily |
| 10 days | 10 days | 10 days | 10 days |
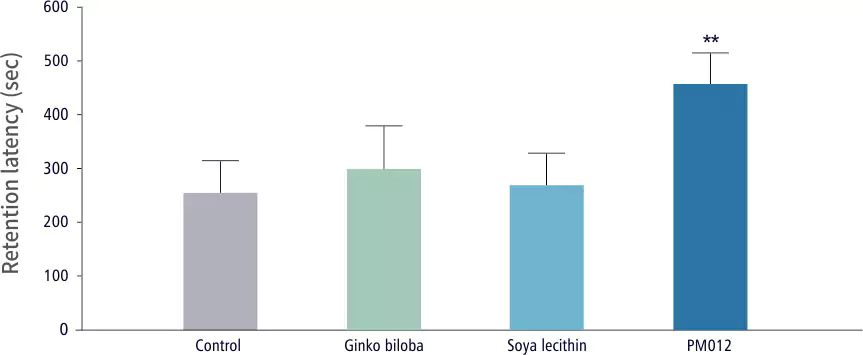
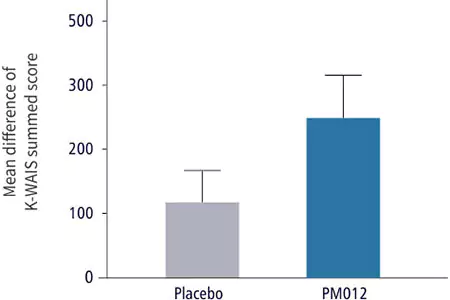
| Cognitive Ability Test | Control Group | Test Group |
| Dosage: | Placebo, 3 tablets/day | 650mg PM-012, 3 tablets/day |
| Duration: | 6 weeks | 6 weeks |
| Confirmation method: | 1. Administered Korean version of Wechsler Adult Intelligence Scale (WAIS), an IQ test | |
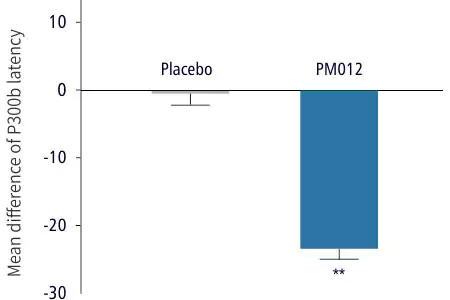
| 2. ERP P300 brain wave measurement: confirmed cognitive function and information processing speed | ||