| List of STDs detected | |||
| Oligo mixture | Oligo mixture (Tube A) | Oligo mixture (Tube B) | Oligo mixture (Tube C) |
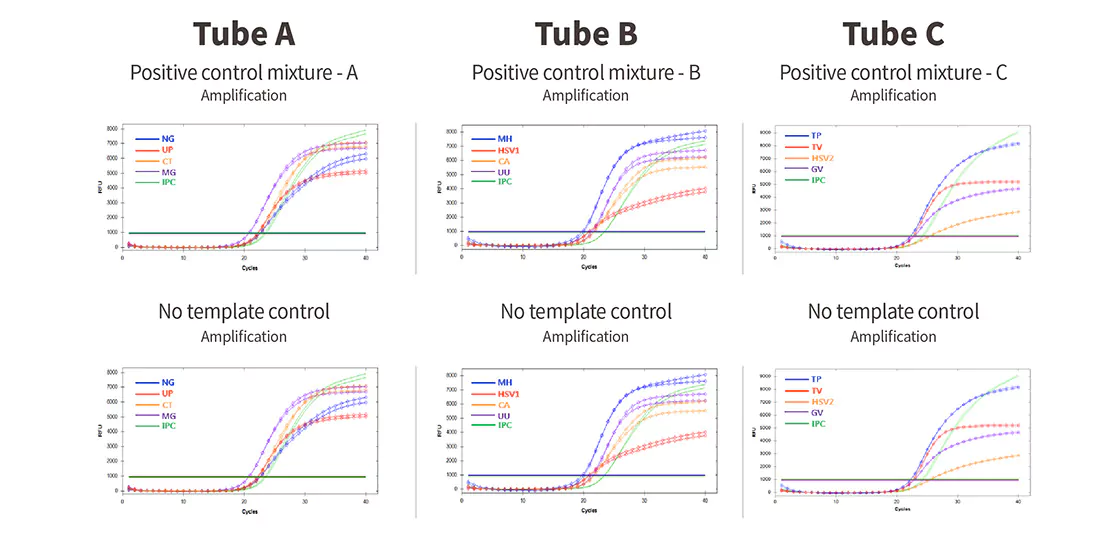
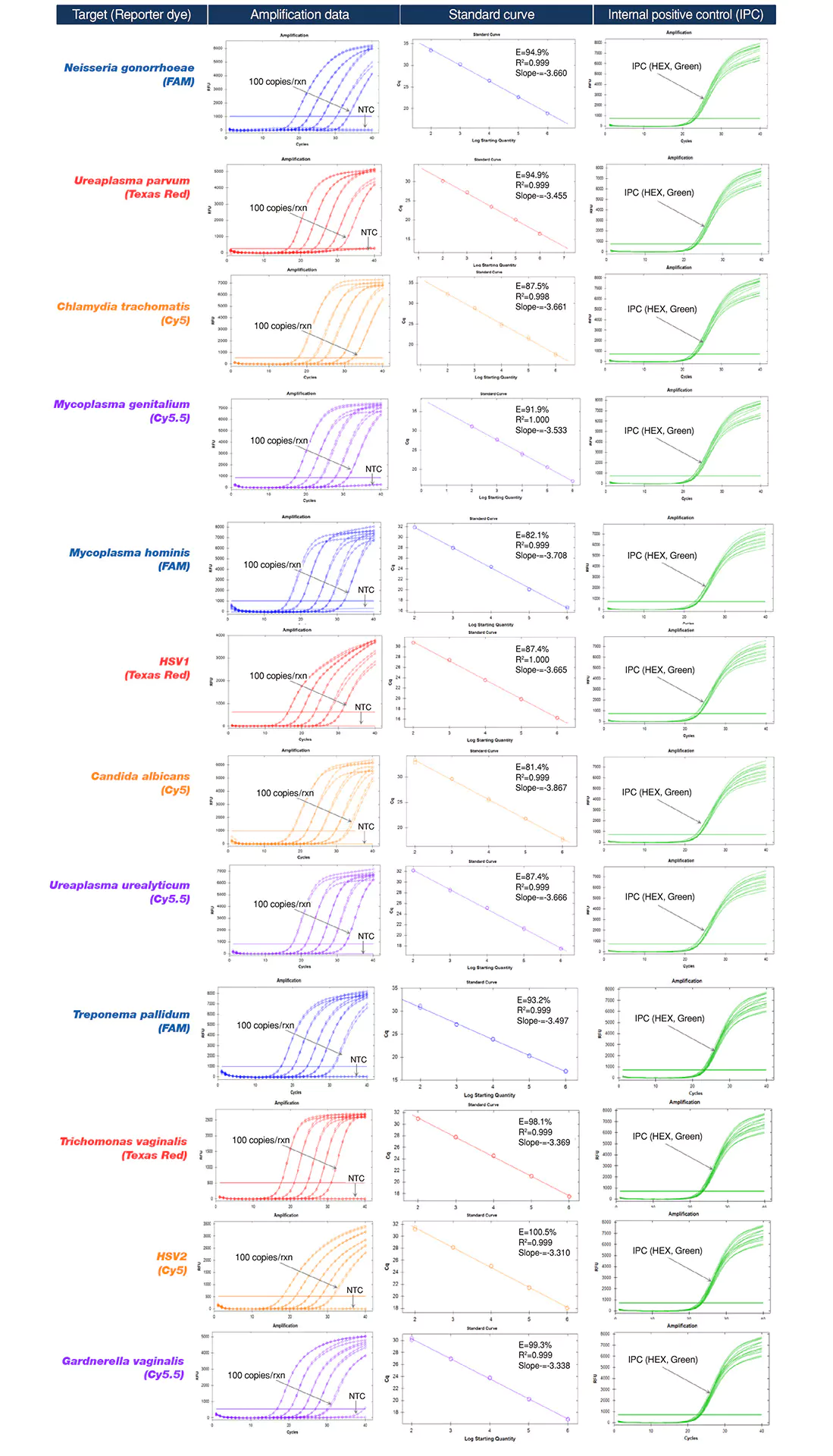
| Pathogens | Chlamydia trachomatis Neisseria gonorrhoeae Ureaplasma parvum Mycoplasma genitalium |
Ureaplasma urealyticum Mycoplasma hominis Candida albicans HSV1 |
Gardnerella vaginalis Treponema pallidum Trichomonas vaginalis HSV2 |
| Compatible specimen types | ||
| Swab specimen | Urine specimen | Liquid based cytology specimen |
| Urethral, vaginal and cervical swab | Standard urine specimen | ThinPrep® and SurePath™ |

| Product Details | ||||
| Product Name | SKU | Test Quantity per Kit | Certifications | Qualifications |
| MF STD12 Kit | MF2003 | 144 individual tests | CE Mark | K-GMP, ISO 13485 |